Leaching gold with a cyanide solution remains the most widely used hydrometallurgical process for extracting gold from ores and concentrates. Despite the challenges and hazards associated with cyanide, few other process have proven to be a more economically viable alternative for large-scale operations.
Chemical Basis of Cyanide Leaching
The chemical reaction that underpins cyanide gold leaching, known as the Elsner Equation, is:
4 Au + 8 NaCN + O₂ + 2 H₂O → 4 Na[Au(CN)₂] + 4 NaOH
In this process, a slurry of ground ore is mixed with cyanide in the presence of activated carbon. The carbon has a strong affinity for the aurocyanide complex and adsorbs the dissolved gold from the solution. Gold loadings on carbon typically range from 1,000 to 4,000 g/t.
Once leaching is complete, the loaded carbon is removed, and the adsorbed gold is stripped under high temperature and pressure using sodium hydroxide and cyanide solutions, forming a high-value electrolyte. Gold bullion is then recovered from the electrolyte through electrowinning.
Key Factors Influencing Cyanide Leaching
Several factors determine the success of cyanide leaching in a mineral processing plant:
- Gold concentration – Assessing whether the gold content in the ore is sufficient to justify extraction costs is essential. Gold projects can be viable at grades as low as 0.5 g/t, depending on economic and processing factors. For lower-grade ores, beneficiation methods such as flotation are often used before leaching to upgrade the ore and reduce the volume requiring treatment.
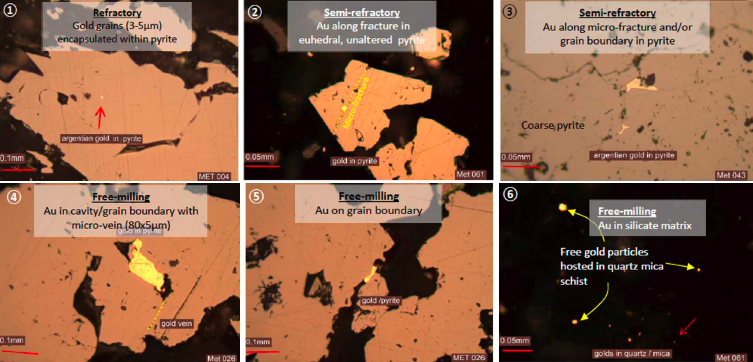
- Nature of gold – The dissolution rate of gold in cyanide is approximately 0.2–0.5 µm per hour. Large gold particles and nuggets may not fully dissolve within standard processing times, making gravity separation methods a more effective recovery option. Cyanidation is primarily used for gold that is finely distributed within a mineral matrix.
- Liberation – For effective cyanide leaching, cyanide must be able to contact the gold particles. This means the ore must be sufficiently porous or finely ground to ensure adequate gold exposure. The grind size and mineral liberation are crucial for optimising gold recovery rates.
- Mineralogy – The presence of certain sulphide minerals can significantly impact cyanide leaching. For example, pyrrhotite can oxidise during leaching, consuming oxygen and generating acid. This reaction can reduce leaching efficiency and may require additional aeration, oxygenation, or neutralisation strategies to prevent the formation of hydrogen cyanide gas. Understanding the ore’s mineral impurities is essential to avoid processing challenges.
Conclusion
Cyanide leaching remains the dominant method for gold extraction, but its effectiveness depends on multiple factors, including gold concentration, particle size, mineralogy, and leaching conditions. A thorough understanding of these elements is essential for optimising gold heap leaching, hydrometallurgical processing, and gold recovery in modern metallurgical testwork programs.
For more information on metallurgical testing services for gold leaching and process optimisation, contact Core Resources today.